A - Decarboxilyation of sodium acetate by NaOH
Sodium acetate undergoes decarboxylation to form methane (CH4) under forcing conditions, pyrolysis in the presence of sodium hydroxide

B- Combustion of Methane
The oxygen (O2) is being reduced, so it is the oxidizing agent. Methane (CH4) is being oxidized, so it is the reducing agent
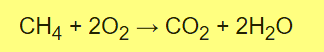
So I prepare Reduzing agent (CH4) from sodium Acetate