:The chemical formula of Ethylamine is
CH3CH2NH2
:The chemical formula of n-propyl amine is
CH3CH2CH2NH2
?How can you prepare ethylamine from n-propyl amine
a- Oxidation of n-propylamine by KMnO4
In presence of an base n-propylamine is oxidized to give Propanal
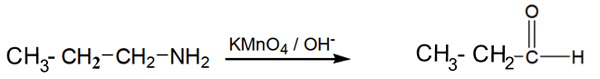
b- Oxidation of Propanal in acidic conditions
Aldehydes are readily oxidized to carboxylic acids
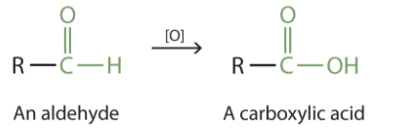
CH3CH2CHO → CH3CH2COOH
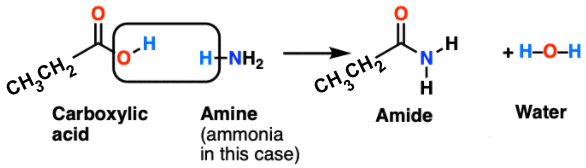
C- Reaction between Propionic acid and ammoina to give amide
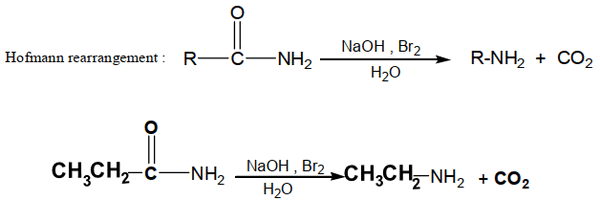
d- Hofmann Rearrangement
Amides with no substituent on the nitrogen react with solutions of bromine or chlorine in sodium hydroxide to yield amines through loss of their carbonyl carbon by a reaction known as the Hofmann rearrangement