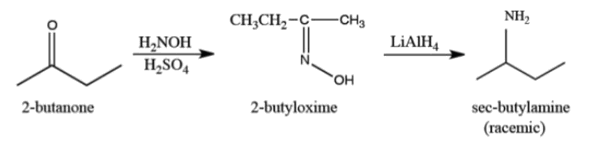
The chemical formula of propyne is
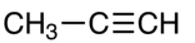
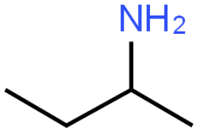
The chemical formula of 2-aminobutane is
?From Propyne how can you prepare 2-aminobutane
a) Reaction of propyne with NaNH2)
One of the major differences between the chemistry of alkynes and that of alkenes or alkanes is that a hydrogen bonded to a triply bonded carbon atom of a terminal alkyne is sufficiently acidic that it can be removed by a strong base, such as sodium amide NaNH2 to give an propynide anion
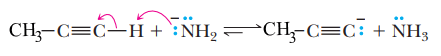
b) Reaction of propynide anion with Haloalkane)
an propynide anion is a strong base. An propynide anion is also a nucleophile; it has an unshared pair of electrons that it can donate to another atom to form a new covalent bond. In this instance, an propynide anion donates its unshared pair of electrons to the carbon of a methyl or primary haloalkane, and, in so doing, the propynide nucleophile replaces the halogen atom. This type of reaction is called a nucleophilic substitution. For example, treating sodium propynide with 1-bromomethane gives 2-butyne

An important feature of this reaction is that new carbon-carbon bonds are made, allowing the construction of larger carbon backbones from smaller ones
c) Reduction of 2-butyne by Hydrogen)
Reduction of 2-butyne by one mole of Hydrogen in presence of pt as catalyst
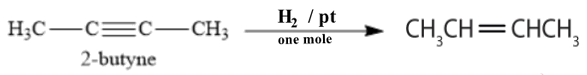
d) Addition water to 2-butene)
But-2-ene is an unsatrated compound with one double bond. . It undergoes addition reaction with water molecule when treated with acidulated water forming butan-2-ol
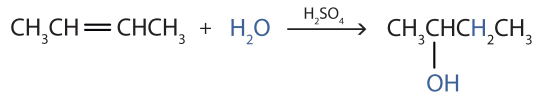
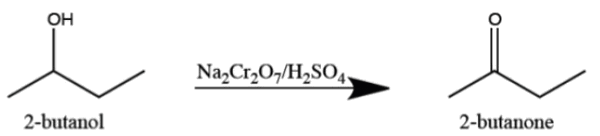
E) Oxidation of 2-butene)
But-2-ene is oxidised by Na2Cr2O7 in presence of H2SO4 forming 2-butanone
F) Reductive Amination)