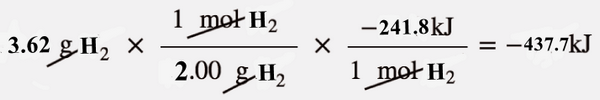:Given The Thermochemical equation
H2(g) + 1⁄2 O2(g) → H2O(g) ΔH = -241.8 kJ/mol
1mol H2 ⇔ 1/2 mol O2 ⇔ 1 mol H2O ⇔ −241.8 kJ
The balanced thermochemical equation relates the energy change to moles, not grams, so we first convert the amount of H2 to moles and then use the thermochemical equation to determine the energy change
Calculation of No of Moles of Hydrogen
1mol H2 → 2 g
mol H2 → 3.62 g ??
no of moles of H2 = 1.81 mol
Calculation of Heat Change
1mol H2 → −241.8 kJ
1.81mol H2 → ?? kJ
Heat Change = - 437.7 kJ
Fast solution