- الترتيب يكون كما بالشكل :
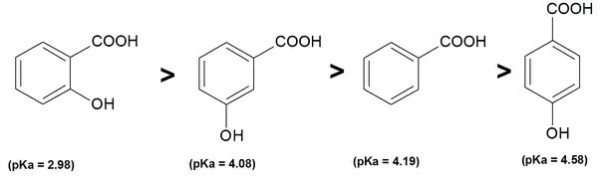
a- O-hydroxy benzoic acid is the strongest acid here
The OH in salicylic acid (the ortho-derivative) will stabilize the anion due to hydrogen bonding
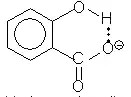
(Hydrogen bonding is also possible in the protonated form (the acid) but is stronger in the carboxylate anion
b- m-hydroxy benzoic acid is more acidic than p-hydroxy benzoic acid
OH group at para position has dominant -M effect (mesomeric effect) but has -I effect (inductive effect) also while OH group at meta position on benzene ring has only -I effect
When both acids donate their proton from -COOH group then p-hydroxybenzoate is more stabilized due to -M effect of nitro group than m-hydroxybenzoate because -M group more deactivates the ring / decreases charge than -I group
Thus, p-hydroxybenzoic acid is more acidic than m-hydroxybenzoic acid